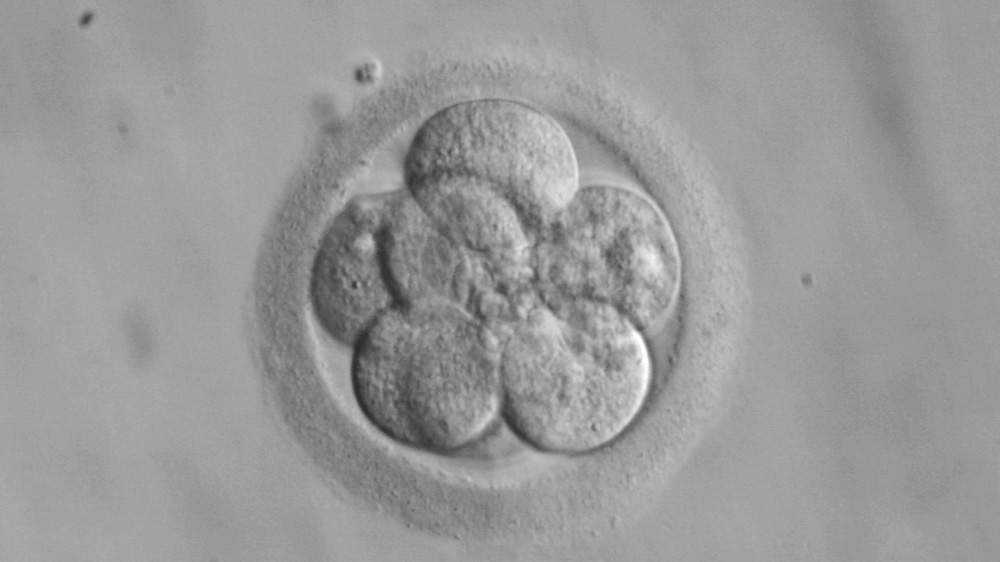
14-Day Rule
Where do we draw the line between a cluster of cells and a human being? The ‘14-day rule’ concerning embryo research is one result of a long process to determine just that.
“I don’t think that I succeeded altogether in getting members of the committee, let alone members of Parliament, to grasp that this was not a question of fact (‘When does life begin?’, as they persisted in asking) but a question that had to be decided. How ought we to regard this new entity, the live human embryo outside the uterus?” (Mary Warnock, quoted in Hurlbut et al. 2017: 1041).
It was first proposed in 1979 by the Ethics Advisory Board (EAB) of the US Department of Health, but became more influential and recognized after the work of the United Kingdom’s Warnock Committee in 1984. The Committee was tasked with dealing with the profound moral uncertainty created by the possibilities raised by then-recent British scientific achievement of in vitro fertilization (IVF), which culminated in the birth of the world’s first ‘test tube’ baby, Louise Joy Brown, in 1978. Moral philosopher Mary Warnock, the Committee´s head, identified the need for a compromise that would satisfy differing points of view on when in vitro embryos could be research objects and when they warranted protection. At the time this line was drawn, research beyond the two-week limit was a technical impossibility.
Decades later, in 2016, new technical developments enabling longer periods of human embryo culture put pressure on the 14-day rule. The International Society for Stem Cell Research (ISSCR), the professional voice of the stem cell scientific community, updated its embryo research guidelines to no longer endorse the 14-day limit in May 2021.
Questions about the value, beginning and limits of human life remain relevant, just as they were at the time the first deliberations took place in the EAB and Warnock Committee. The promise of therapies and treatments is not sufficient to justify what some may see as a violation of the sanctity and dignity of human life. Removing limits in light of new technical developments suggests that the research community is willing to entertain societal limitations on their research only as long as they do not practically restrict their research agendas.
Changing the result of both scientific and political agreement should involve steps equivalent to those that led to the establishment of the 14-day rule in the first place: an extensive and inclusive deliberative process.
The questions raised by reproductive technologies: a new biological entity
In vitro fertilization (IVF) and new reproductive technologies raised important questions about human life when they were introduced in the 1970s, including the status of family relationships and the risks of technological interventions for new babies. How to handle a new entity, the in vitro human embryo, became a crucial question without clear answers. Its use seemed justified for reproductive purposes and fulfilling the dreams of would-be parents, but could it become an object of laboratory research too? That is, should the law permit research with human embryos? IVF involved the production of more embryos than would be used for starting a pregnancy, and scientists considered these embryos valuable for biomedical research opportunities; otherwise, they would be discarded or kept frozen in clinics. This put pressure on resolving pressing ethical questions: As a potential human, does an embryo deserve the same rights and protections as a human person? If so, should these rights and protections begin at the point of conception, or at some later point?
Questions of the moral status of the embryo have once again become unsettled, requiring new processes of democratic deliberation and negotiation among different points of view widely held in society. Far from being scientific questions alone, deciding what policies should handle embryo research incorporate other elements that include trust in science and multiple moral points of view. While the 14-day rule as it originated in the UK has seen broad adoption internationally, different countries have also addressed questions of embryo research in different ways, according to their own political cultures and ways of public reasoning (Jasanoff, 2005).
Origins of the 14-day rule
On the heels of the landmark Roe v. Wade United States Supreme Court decision and in the wake of advances in reproductive medicine, the Ethics Advisory Board (EAB) of the Department of Health, Education, and Welfare (HEW) (1978-1980) was created to address regulatory and ethical issues surrounding IVF. One important question facing the EAB was whether embryos created for IVF could be repurposed as research objects. Using justifications from previous regulations and developmental biology, the EAB recommended limiting research on embryos to 14 days after fertilization (EAB, 1979).
This timeframe aligned with previously established federal regulations that were based on the impossibility of detecting the presence of the conceptus (placenta, membranes, and embryo) in vivo before 14 days. Notably, the EAB did not make an ethical judgment when establishing the 14-day rule, nor did it provide ethical justification. The rule was instead seen as a convenient policy response to an ethical concern. As the selection of the 14-day timeframe was pragmatic and largely arbitrary, it avoided the need to assume an ethical standpoint. The restrictions the EAB placed were a compromise to gain approval within the committee and respect differing views. Thus, the distinction of the moral status of the embryo was made a matter of developmental biology, with ethical and religious claims taking a backseat to scientific claims (Hurlbut, 2017). Though this recommendation was the first version of a ‘14-day rule,’ the EAB’s recommendations were never implemented. The version of the 14-day rule that eventually saw broad adoption was arrived at independently a few years later across the Atlantic.
Assisted reproduction was widely welcome in Britain throughout the 1970s. The first baby conceived via in vitro fertilization (IVF), Louise Joy Brown, was born on July 25, 1978 in Manchester. This marked the culmination of the work of Cambridge physiologist Robert Edwards and cemented the UK’s status as a biotechnology pioneer. By the turn of the decade, public opposition and concerns about IVF had grown; by 1980, Members of Parliament were lobbying for a governmental inquiry. The government established an expert committee in 1982 that was not restricted to scientific experts, as was the norm at the time. Moral philosopher Mary Warnock chaired the committee, which accordingly came to be known as the Warnock Committee. Warnock would mark a shift in bioethics to address public sentiment and morality (Wilson, 2014).
The Committee released a report in 1984 that became known as the Warnock report. It became famous for establishing the 14-day rule for the time limit an in vitro embryo could be kept alive and used for experimentation purposes. The committee took into consideration broad public input and ample consultation with concerned parties (Hammond-Browning, 2015). In Mary Warnock’s words, the committee sought to avoid a “slippery slope” by firmly establishing a line that should not be crossed or moved (Hurlbut et al., 2017). The committee understood that public trust required finding a compromise to structure regulation that was grounded not on science alone, but also on common-sense understanding and moral reasoning. Still, biology did play a role in defining the 14-day limit thanks to Anne McLaren, the only research scientist serving on the Warnock Committee. Around the two-week point in embryo development, the so-called “primitive streak” appears. The streak is a precursor to the nervous system, marks the start of cellular differentiation, and marks the point after which twinning becomes impossible. Individuality thus has been a key feature of conceptualizations of moral value (Steinbock, 2009).
The committee did not suggest identifying the emergence of the primitive streak as a criterion, but emphasized a time limit, which lent to its success: following a timeline can be easily monitored and enforced. Reflecting on the subject, Warnock recalled that the timeline was practical: “I chose 14, rather than 13 or 15, simply because everyone can count up to 14; a fortnight is a good, memorable number, and records can be kept week by week” (Hurlbut et al. 2017, 1041). Besides workability, the committee did not seek an answer for when human life begins or what is the moral status of the embryo. Instead, the committee provided the human embryo a ‘special status,’ without defining its moral status (Warnock, 1987). The success of the recommendation reflected a compromise between biological knowledge, morality, common sense, and pragmatism (link: Warnock 2017). For Director Sheila Jasanoff, Warnock succeeded in recognizing that the embryo was an ‘ambiguous new entity,’ whose ‘moral status had to be determined for society through an authorized form of deliberation. This was at bottom a moral and even a legislative question, one for society to decide.’ (Jasanoff, 2019). The drawing of distinctions in embryo development and novel ontological categories owes to an empiricist culture in Britain and a commitment to establishing limits for research (Jasanoff, 2005).
The Warnock Report’s publication was not without controversy. It was met with concerns about loss of public trust in science and impairing potentially beneficial research. It also received criticism for its utilitarian approach that did not reach moral consensus on a philosophical basis, as well as for ignoring the ‘potential’ of the embryo to become human (Hammond-Browning, 2015). Its justifications for the 14-day limit are still debated, with some scholars finding it problematic and suggesting alternative criteria such as sentience or the point at which an embryo could feel pain (Castelyn, 2020).
Six years of deliberation and debates in the British parliament followed the Warnock Report in the UK, during which a total ban on embryo research was contemplated. In the meantime, the British government released in 1987 the white paper ‘Human Fertilisation and Embryology’ (Department of Health and Social Security, 1987), which laid the ground for fertility legislation. From these debates, the novel category of the “pre-embryo” emerged, alongside the 1990 legislation that finally enshrined the 14-day rule (Mulkay 1997; Human Fertilisation and Embryology Act 1990). Mary Warnock ultimately had sufficient legitimacy and authority to perform an act of ‘ontological surgery’ (Jasanoff, 2011), defining the limits and uses of life itself. The British government created an organization to regulate infertility treatments and embryological research (one of the recommendations of the Warnock report), the Human Fertilisation and Embryology Authority (HFEA). Since its formulation, the 14-day rule has been widely embraced as either a strict legal restriction on research or an official guideline. However it has not been universally adopted, and regulations vary from total prohibition on human embryo research to complete lack of national law or guidelines (Matthews and Moralí, 2020; Matthews et al., 2021).
The 14-day rule has dominated the embryo policy landscape for more than three decades since the Warnock Report. It owes its legacy largely to the understanding that science alone cannot settle moral disputes (Chan 2018). Accordingly, the Report also reinforced the need of external oversight of medical and scientific activities (Wilson, 2014). Warnock recognized that moral disagreement was unavoidable and that practical compromise was the best way forward; such “ontological and political reality was not a product of biological knowledge alone but was created (or co-produced) out of a complex mix of pragmatism, empiricism, and trust in experts” (link: Jasanoff, 2005). The form of moral pluralism that the committee evinced would shape the discipline of bioethics for years to come (Wilson, 2011). While technologies and social attitudes may change over time, the central questions the committee dealt with, such as under what conditions is it appropriate to manipulate the course of human embryonic development, remain with us. As ever, science alone cannot provide the answers.
The 2021 update to ISSCR guidelines
Lack of scientific advances in embryo culture have accounted for the relative lack of debate regarding the extension of the 14-day rule since its inception. However, nearly forty years after the publication of the Warnock report, in 2016, scientists in the UK and the US reported the capability of maintaining and culturing living human embryos for longer periods than 14 days (link: Shahbazi et al., 2016; Deglincerti, 2016; see Rossant, 2016, for an explanation of these advances). On May 26, 2021, the International Society for Stem Cell Research (ISSCR) updated its 2016 guidelines (link: ISSCR 2016). The new guidance (link: ISSCR 2021) relaxes the 14-day limit on embryo culture and suggests evaluating research on embryos past the two-week limit on a case-by-case basis, with several phases of review (Anthony et al, 2021). This new guidance came as the result of an 18-month procedural deliberation carried out by a taskforce of 45 international experts, scientists, clinicians, ethicists, lawyers, and policy specialists. The group, chaired by British stem cell biologist Robin Lovell-Badge, sought input from polls and public-engagement activities and submitted their revised guidelines to an additional set of experts for peer review. Researchers close to ISSCR argued it was “time for an update” (Lovell-Badge 2021) and time to revive public conversations around this set of issues (Clark et al., 2021). Accordingly, the committee called for stakeholders to lead public conversations about how to respond to new scientific possibilities (Mummery and Anthony, 2021).
The revised guidelines emerge as some question whether the 14-day rule still meets its original purpose (link: Appleby, 2018) or whether it obstructs the ‘human right to benefit from science’ (Master et al., 2021). These critics of the rule see a lack of compelling reasons to maintain it in light of potential biomedical benefits (McCully, 2021). Others have argued that the revision of the 14-day limit should proceed according to incremental steps (Hyun et al., 2021) and considered embryo regulation a dynamic process that should adjust to changing tides. They point to the practical–but not moral–consensus achieved by the Warnock Committee as evidence (Chan, 2017). Even before the 2016 breakthroughs in embryo culture, some thought advances in reproductive technologies merited a new ‘Warnock-style committee’ to update its vision of governance (Hammond-Browning, 2015).
The updated ISSCR guidelines have met prompt criticism from many in the bioethics community who defend the 14-day rule and take issue both with its refusal to establish limits on embryo research and its broad lack of engagement with moral concerns (Johnston et al., 2021; Baylis, 2021; Blackshaw and Rodger, 2021; Peters, 2021). Even if the 14-day rule is relaxed, there is no clear consensus on what sort of policies would replace its many international implementations or what kind of oversight would be required (link: Sawai et al., 2021; Matthews et al., 2021).
The Observatory’s Point of View
The 1979 EAB report (EAB, 1979) began its conclusions, “It is now technically possible to fertilize a human egg outside the body of a woman and then transfer the fertilized egg” (p. 100), suggesting it provided a response to recent scientific advances. The idea that it is now “time for an update” to the 14-day rule is in a similarly reactive mode. But should technological change be the driver of moral deliberation? Embryo policy is constituted around a technological and cultural object. Still, the idea that society should react to scientific developments has become a deeply seated narrative of governance in advanced nations. For instance, Appleby and Bredenoord (2018) write that ‘science is changing, and regulations need to adapt.’ In this arrangement, ethics and law lag behind the progress of science (Jasanoff, 2019).
Those in favor of extending the 14-day rule justify their position by arguing that studying later-stage embryos would pave the way for novel therapeutics and other biomedical interventions. However, the promise of therapies and treatments is not sufficient to toss moral reasoning aside, and allowing research to continue without sufficient consideration of its moral dimensions sets a dangerous precedent. In the case of embryo research, extending the 14-day rule could lead to unexpected ramifications, such as facilitating the advance of human germline genome editing technologies (Nuffield Council on Bioethics, 2018). Allowing research to continue if scientists can argue for its benefits risks overstepping moral reasoning. Establishing controls reassures the public that scientists are willing to keep their creations and experiments in check for the public good. A willingness to depart from previously established limits may raise suspicion about scientists governing themselves (Foht, 2021). If the rule is extended by virtue of new technological achievements, this signals that scientists have complied with the limit only because it was not technically possible to overcome the limit in the first place. As Observatory Co-Director Ben Hurlbut notes, “If you abandoned every rule or law that inhibits you as soon as it inhibits you, we’d live in a lawless world.”
The Warnock Report has stood the test of time partly due to its compromise and respect for competing views concerning the moral status of the embryo. Mary Warnock reminds us that ‘this was not a question of fact (‘When does life begin?’, as they persisted in asking) but a question that had to be decided. How ought we to regard this new entity, the live human embryo outside the uterus?’ (Mary Warnock, quoted in Hurlbut et al. 2017: 1041). The questions that were relevant then remain relevant in the face of new scientific knowledge. Members of the public have legitimate reasons to think differently about the value and treatment that embryos deserve. Producing more evidence or facts should not be expected to simply lead the public to favor embryo research (Cavaliere 2017). As Hyun and colleagues explain, the 14-day rule “was never intended to be a bright line denoting the onset of moral status in human embryos. Rather, it is a public policy tool designed to carve out a space for scientific inquiry and simultaneously show respect for the diverse views on human-embryo research” (Hyun et al., 2016: 170). Answers to the questions of political acceptability, desirable courses of action, and when human life starts require such respect for diverse views and cannot be found in the laboratory.
Changing the result of both scientific and political agreement should involve equivalent processes that led to the establishment of the 14-day rule in the first place: an extensive deliberative and inclusive process about whether the public is in favor or against the extension of the 14-day limit for embryo research. This is part of the broader work of developing more robust capacities for deliberation and governance that allow the public to shape and decide the role of science and technology in the societies we want to live in. Extending the 14-day rule without developing such processes risks eroding hard-earned trust in scientists as custodians of the ethical views that societies deposit in the regulation of their practices.
For Observatory Co-Director Ben Hurlbut, questions about extending the 14-day rule “must be asked in ways that acknowledge the moral concerns of the wider social community” (Hurlbut et al. 2017: 1032). Science should be subordinate to society’s judgments, values, and preferences, not vice versa. Though the ISSCR claims to have undergone an extensive process of deliberation and consultation, the framing and limitations of its processes bear further scrutiny. The 14-day rule established a norm of social acceptability for scientific research that was not a product of biological knowledge alone but was created out of a complex mix of pragmatism, empiricism, and trust in experts. As scientific prowess increases so must democratic mechanisms for representation and participation that ensure research is conducted within the realm of what societies find morally permissible. Given that technological developments have spurred reevaluation of the rule, the Observatory will remain attentive to the unfolding of these processes and the imaginaries of governance that animate them.